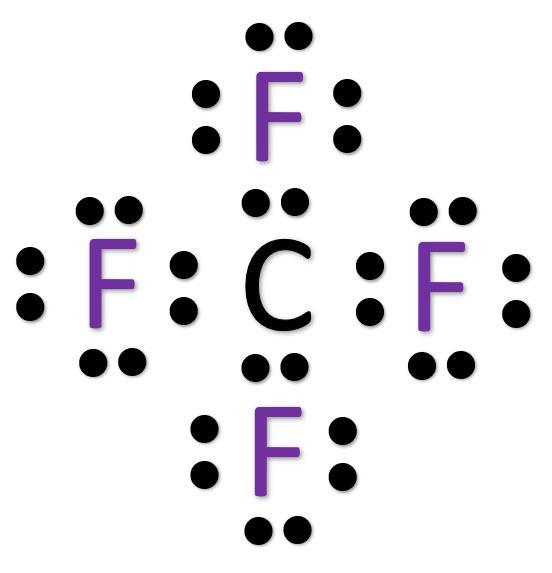
To create the Lewis structure we need to take into account the octet rule: atoms tend to gain, lose or share electrons to complete their valence shell with 8 electrons.
C belongs to Group 4A in the periodic table so it has 4 valence electrons. It needs to share 4 pairs of electrons to complete the octet.
F belongs to Group 7A in the periodic table so it has 7 valence electrons. Each F needs to share 1 pair of electrons to complete the octet.
As a consequence, in CF₄, C will form a single bond with each F and all the octets will be complete.